CLEARLine® Serological pipettes are transparent polystyrene, available in volumes of 1 ml, 2 ml, 5 ml, 10 ml, 25 ml, 50 ml, 100 ml*.
These pipettes are designed to be used with electronic or manual pipettors to transfer liquids of various volumes within laboratory
operations.
The presence of bidirectional graduations and negative graduations contributes to enhancing the precision of the pipetting operation.
The color-coded system associated with different volumes makes the pipettes easily identifiable.
Each pipette is equipped with a filter designed to prevent the accidental aspiration of liquids or foreign materials, thus protecting both
the pipette and the sample from contamination.
Sale Unit: 1000
Details DatasheetSale Unit: 1000

Sale Unit: 800
DetailsSale Unit: 800
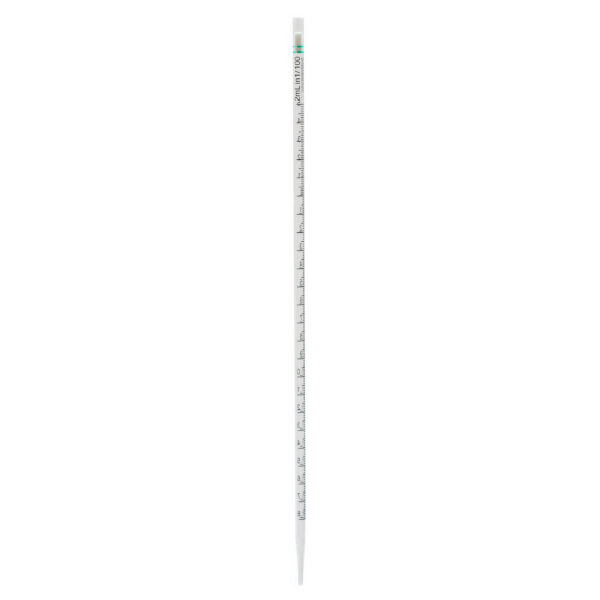
Sale Unit: 300
DetailsSale Unit: 300
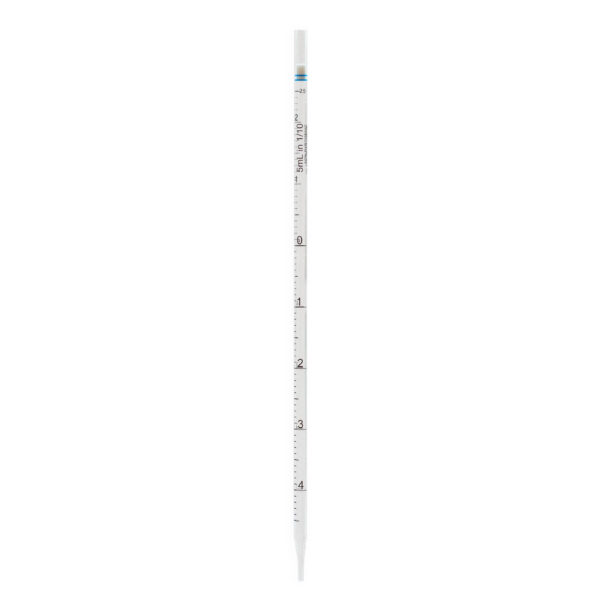
Sale Unit: 200
DetailsSale Unit: 200
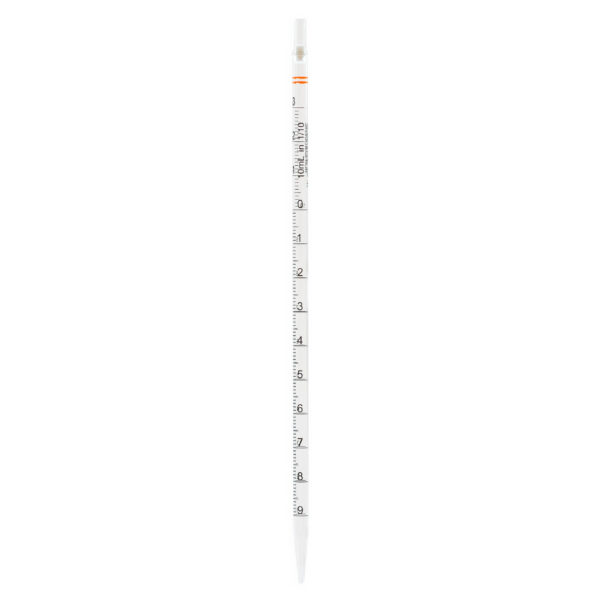
Sale Unit: 200
DetailsSale Unit: 200
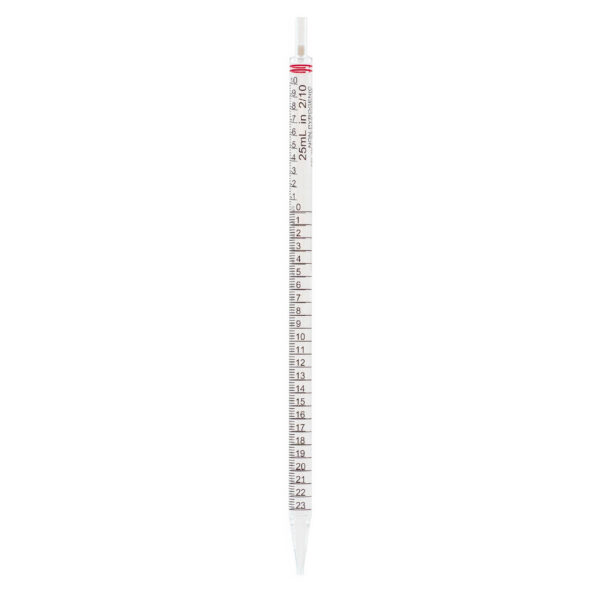
Sale Unit: 100
DetailsSale Unit: 100

Sale Unit: 50
DetailsSale Unit: 50

Sale Unit: 1000
DetailsSale Unit: 1000

Sale Unit: 800
DetailsSale Unit: 800

Sale Unit: 500
DetailsSale Unit: 500

Sale Unit: 350
DetailsSale Unit: 350

Sale Unit: 350
DetailsSale Unit: 350

Sale Unit: 100
DetailsSale Unit: 100

Sale Unit: 50
DetailsSale Unit: 50

Experience, reliability and innovation a your service
Since 1988 Biosigma S.p.A. produces and distributes disposable plastic items for biotechnology, research, clinical chemistry and pharmaceutical laboratories. In our over 12.000 sqm facilities, we dispose of latest injection machines and informatics tools that allow production of high standard quality articles still being competitive on the worldwide market.
© 2024 Biosigma S.p.A. – VAT ID: IT03328440270 – All rights are reserved.
BIOSIGMA S.p.A. – Via Valletta, 6 – 30010 – Cona (Venice, Italy) | Exp. Dep. Direct No.:+39 0426 302226
Made with ♡ by RSW Studio